About
Research Interests
We are interested in understanding the basic cellular mechanisms of neurodegeneration in Alzheimer’s disease, frontotemporal dementia, chronic traumatic encephalopathy and other neurodegenerative disorders. The ultimate goal is to translate relevant findings into therapies for our patients. Novel approaches are needed given the large number of clinical trial failures in the neurodegenerative disease field. Current studies in the lab include:
Basic Science Research
-
Astrocyte changes in FTD and other neurodegenerative disorders - Proteomic studies demonstrated prominent enrichment of astrocyte proteins in FTD-MAPT cases, even more than observed in AD. It is unclear if this represents nonspecific inflammation or perhaps compensatory mechanisms in the brain to manage pathologic proteins. Subsets of astrocyte proteins, including end-feet may play an important role in the pathophysiology of disease. We are trying to understand how these findings impact the pathophysiology of disease.
-
Proteomic sequencing of AD, FTD and CTE - We will often rely on proteomic sequencing of postmortem human brain to identify proteins that may be involved the process of neurodegeneration. Thanks to previous funding from the Department of Defense, we sequenced increasing stages of CTE for comparison with other tauopathies so as to understand the molecular overlaps and to identify potential modifiers of tau. We are also working on a dataset comparing specific change in grey and white matter across tauopathies. Further validation of significant targets continues to be a goal as we move into the future.
-
Dermal fibroblasts as a surrogate measure of neurodegeneration. Thanks to funding from the the BrightFocus Foundation, we are exploring proteomic changes in fibroblasts in response to stressors from subjects with various neurodegenerative disorders. In a collaborative project we are also exploring neurodegeneration associated proteins in skin samples as biomarkers of disease.
-
Utilizing human induced pluripotent stem cells (IPSc) to understand how microtubule protein tau (MAPT) genetic mutations cause frontotemporal dementia - Mutations in the MAPT gene can lead to frontotemporal dementia however it remains unclear as to how these mutations ultimate lead to neurodegeneration. The goal of this project is to utilize differentiated neurons developed from IPS cells and other cellular and biochemical techniques to identify dysfunctional cellular pathways that may contribute to the disease process. IPS cells are generate in the lab from fibroblasts that are collected from research volunteers who harbor MAPT genetic mutations. We are especially interested in MAPT mutations P301L and R406W. Genomic editing techniques (CRISPR/Cas9) are being explored to generate isogenic lines for comparative study. We are also interested in ‘aging’ differentiated cell lines so as to better recapitulate neurodegenerative changes. The ultimate goal is to identify human relevant targets that can be translated into therapies for our patients.
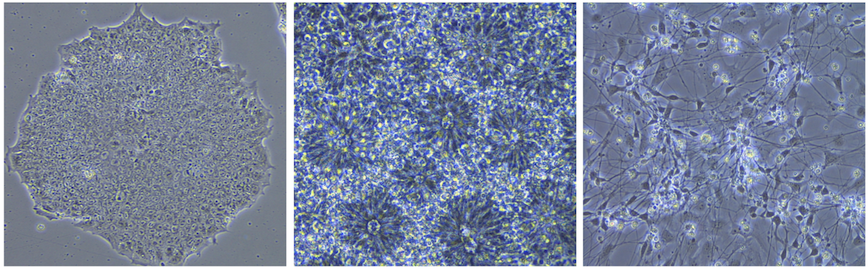
-
Related changes across various neurodegenerative disorders There are likely common mechanisms that are important for many of the primary neurodegenerative disorders. As a result, we have collected fibroblast samples for generating IPS cells from many different research subjects for comparative analysis in the above mentioned assays. Diseases include Alzheimer’s disease, frontotemporal dementia, ALS, Parkinson’s disease, and autism.
Clinical research
- Clinical trials and observational studies As part of the Goizueta Alzheimer’s Disease Research Center, Dr. Hales serves as sub-investigator or primary investigator of several trials. Current studies include evaluating an anti-tau antibody in Alzheimer’s disease and a novel therapeutic for patients who have frontotemporal dementia and an underlying mutation in the progranulin gene. We are also working towards being approved as a site for the ALLFTD consortium.
Previous studies and interests
-
RNA processing dysfunction in AD - Studies identified aggregates of small nuclear ribonucleoproteins (snRNPs) in AD in addition to dysfunctional RNA splicing. Studies utilizing traditional biochemical and cell culture techniques in addition to more advance proteomic and RNA sequencing were utilized to understanding the role of dysfunctional RNA splicing in AD. These studies were also aimed at better understanding the role of RNA processing dysfunction in chronic traumatic encephalopathy (CTE) and the overlap with AD as well as how RNA processing factors may contribute to the aggregation of tau.
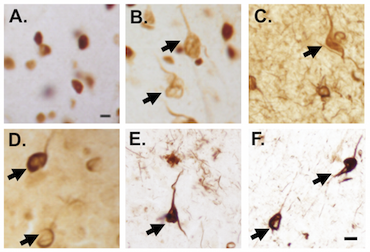
-
Understanding extracellular neuronal network activity in neurodegeneration - Human cognition is based on the electrical activity generated by billions of neurons divided into distinct functional networks. Neurodegenerative diseases like Alzheimer’s disease and frontotemporal dementia lead to dysfunctional network activity that manifests as impaired cognition. Understanding this process may be helpful in generating new therapies. Therefore one aspect of the lab is devoted to evaluating various neurodegenerative disease models (including primary rodent and human IPSc derived neurons) in dissociated neuronal networks on microelectrode arrays. Our recording system is the Maestro by Axion Biosystems.